- Shanghai Reinovax Biologics Co., Ltd.
-
Tel.: 021-50999002E-mail: info@reinovax.comAddress: 5th floor, Building 1,367 Shengrong Road
Pudong New District, Shanghai, China
Reinovax launched the Phase I Clinical Trial for 24-Valent Pneumococcal Polysaccharide Conjugate Vaccine in Zhenjiang, Jiangsu province.
2023-11-21News
24-valent pneumococcal polysaccharide conjugate vaccine (hereinafter referred to as PCV24), developed by Shanghai Reinovax Biologics with own intellectual property, was initiated Phase I clinical trial in Zhenjiang, Jiangsu province on November 16. As a category I preventive biological product, this PCV24 was approved for clinical trials in China by the National Medical Products Administration (NMPA) on January 12, 2023.
The Phase I clinical trial will enroll 240 participants to evaluate the safety of the vaccine in individuals aged 18 to 60 and those over 61. Under the scientific design and rigorous selection by Professor Zhu Fengcai's team, the first cohort of subjects has been successfully registered. Under the guidance of the clinical trial team, these individuals have completed the initial vaccination as well as safety monitoring post the vaccination.

Pneumococcal Disease (PD) is one of the serious public health issues globally, causing over 1.6 million deaths annually [1]. The World Health Organization (WHO) classifies diseases preventable by vaccines, with PD and malaria being classified as "Very High Priorities" for vaccination [2]. Streptococcus pneumoniae is widely distributed in nature and can cause person-to-person transmission through respiratory droplets or bacteria colonizing the nasopharynx. Vaccination is the most effective means of preventing and controlling the spread of the disease. Although polysaccharide vaccines can elicit specific antibodies, they fall short in several aspects for example, it do not generate immune memory, antibody levels wane over time quickly, it fails to prevent bacterial colonization in the nasopharynx and do not provide herd immunity. In addition, polysaccharide vaccines often provide inadequate immune protection when administered to children with underdeveloped immune systems. In contrast, the conjugate vaccine by conjugating polysaccharides to protein carriers through chemical methods can produce immune memory with the help of T-cells, leading to robust, high-affinity, long-lasting antibody responses. It can not only elicit a strong immune response in adults but also provides excellent immune protection for children and the elderly.
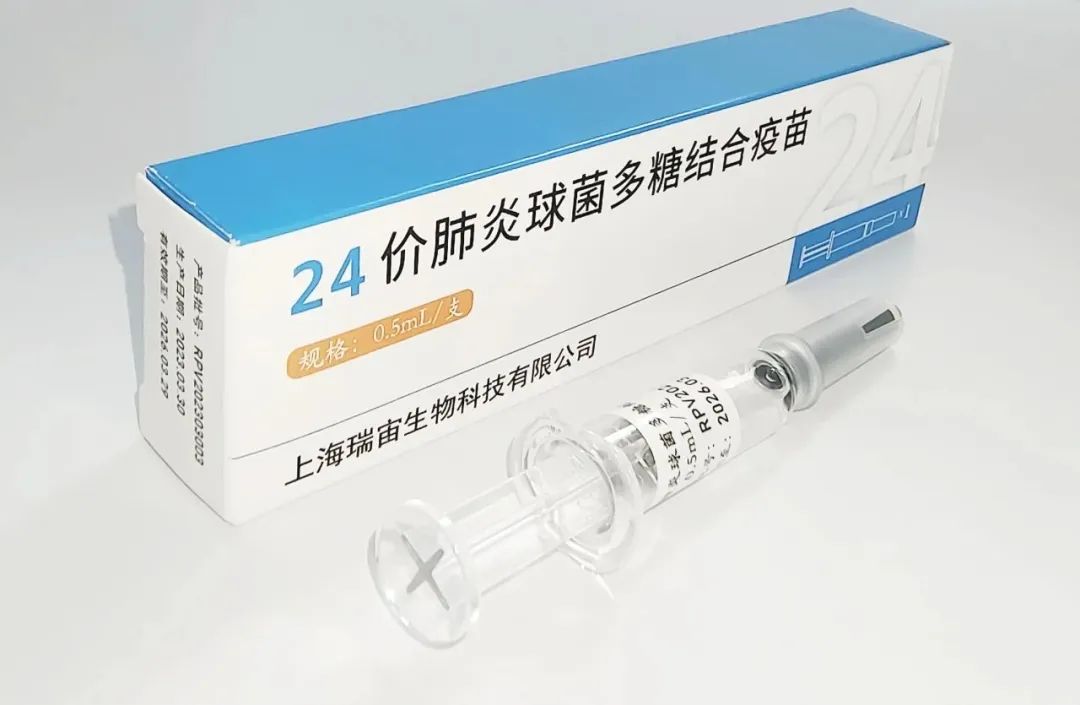
There are nearly 100 serotypes of Streptococcus pneumoniae found in nature [3]. The available PCV13 on the market can cover about 70% of the serotypes in China [4], and there is an urgent need for higher-valent pneumococcal conjugate vaccines to cover a broader range of serotypes. “24-valent pneumococcal polysaccharide conjugate vaccine with dual-carrier proteins” developed by Reinovax Biologics is the first PCV24 vaccine using non-toxic dual-carrier proteins in the world. The vaccine employs Reinovax’s proprietary carrier protein technology which leads to higher safety and simpler manufacturing process. In addition to the adult indications in this trial, Reinovax plans to initiate clinical trials for pediatric indications in early 2024.
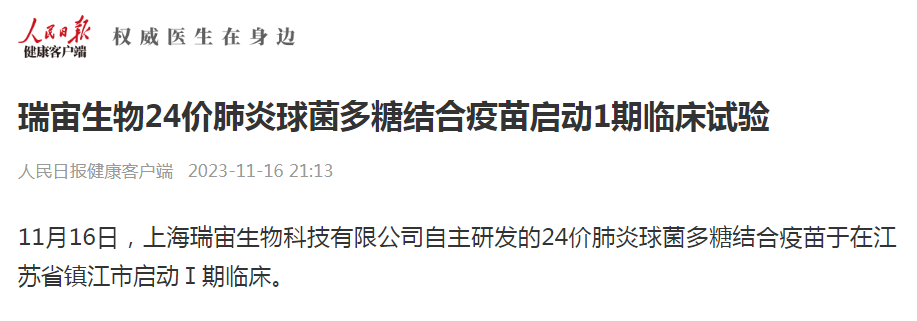
The launch of Reinovax’s PCV24 in the clinical trial has attracted widespread attention with the reports from People's Daily Health Client and Vaccine Circle (click for details).
References
1. Johnson HL, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010 Oct 5;7(10): e1000348. doi: 10.1371/journal.pmed.1000348
2. Meeting of the Immunization Strategic Advisory Group of Experts, November 2007--conclusions and recommendations. Wkly Epidemiol Rec. 2008 Jan 4;83(1):1-15. English, French. PMID: 18175408
3. Masomian M, Ahmad Z, Gew LT, Poh CL. Development of Next Generation Streptococcus pneumoniae Vaccines Conferring Broad Protection. Vaccines (Basel). 2020 Mar 17;8(1):132. doi: 10.3390/vaccines8010132
4. Expert Consensus on Immunoprophylaxis of Pneumococcal Disease (2017 Edition), Chinese Journal of Preventive Medicine, March 2018, Volume 19, Issue 3
News Center
News Recommendation





 Friendship link: Changchun High-tech Group
Friendship link: Changchun High-tech Group