- Shanghai Reinovax Biologics Co., Ltd.
-
Tel.: 021-50999002E-mail: info@reinovax.comAddress: 5th floor, Building 1,367 Shengrong Road
Pudong New District, Shanghai, China
Reinovax Biologics Initiated Phase I Clinical Trial of 24-Valent Pneumococcal Polysaccharide Conjugate Vaccine in Infants and Toddlers
2024-04-26News
The 24-valent pneumococcal polysaccharide conjugate vaccine (PCV24) independently developed by Shanghai Reinovax Biologics initiated the phase I clinical trial for infants and toddlers on April 26th.
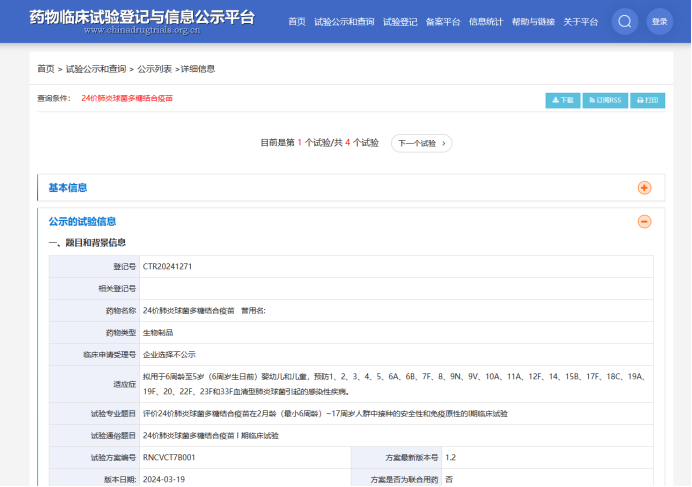
This Phase I clinical trial for infants and toddlers is a randomized, double-blind, parallel-controlled study, planning to enroll 230 subjects. Different age groups will receive the PCV24 vaccine according to corresponding immunization schedules, compared with the 13-valent pneumococcal polysaccharide conjugate vaccine, to evaluate the safety and immunogenicity of PCV24 in a population aged from 2 months (a minimum of six weeks) to 17 years.

Streptococcus pneumoniae is a major pathogen causing severe diseases such as pneumonia, meningitis, and bacteremia in children, and it is also a common cause of acute otitis media and sinusitis. According to the statistics of the World Health Organization (WHO) in 2012, the majority of the pneumococcal disease in children under the age of 5 worldwide took place in Africa and Asia, accounting for 66% of all cases, while China represents 12% of the global cases. Additionally, pneumococcal diseases are one of the leading causes of death among children under the age of 5. The World Health Organization's report in 2019 indicated that approximately 5.83 million children under the age of 5 died worldwide with pneumococcal diseases accounting for 294,000 of those deaths in 2015【1】. Therefore, WHO has classified pneumococcal disease as a vaccine preventable disease at" very high priority
Based on the different pneumococcal capsular polysaccharides, Streptococcus pneumoniae can be divided into over 90 distinct serotypes. Due to the large number of pathogenic serotypes and their varying distribution across different countries, regions, and populations, this poses a significant challenge for the prevention of pneumococcal diseases. Globally, the 13-valent pneumococcal conjugate vaccine (PCV13) is widely used for infant immunization and plays an important role in preventing diseases caused by 13 serotypes included in the vaccine. However, there is a notable increase in the substitution and prevalence of non-vaccine serotypes of S. pneumoniae. For instance, in some countries with high vaccination coverage, the 13 serotypes targeted by PCV13 account for only about 30% of the observed invasive pneumococcal diseases【2】.
The 24-valent pneumococcal polysaccharide conjugate vaccine (PCV24) independently developed by Reinovax Biologics covers polysaccharides from 24 serotypes of pneumococci. By conjugated with carrier proteins, these polysaccharides can significantly enhance the immunogenicity of the polysaccharides. After immunization, PCV24 will induce specific protective antibodies against 24 prevalent serotypes of pneumococci, and it can also generate immune memory through T cells, providing long-lasting immune protection, which offers a strong safeguard against pneumococcal infection.

Reinovax Biologics R&D Center (Located in Zhangjiang, Shanghai)
The Phase I clinical trial for infants and toddlers will include the immunization in the population aged from 2 months to 17 years old, and evaluate the safety and immunogenicity of the 24-valent pneumococcal polysaccharide conjugate vaccine.
News Center
News Recommendation





 Friendship link: Changchun High-tech Group
Friendship link: Changchun High-tech Group